Controlling redox potential during fermentation: groundbreaking work from Roger Boulton and colleagues that deserves some attention
Roger Boulton, emeritus professor from University of California Davis and one of the great authorities on wine chemistry, gave a remarkable presentation at the Australian Wine Industry Technical Conference today on the subject of precision fermentation management. He began by pointing out that there are two issues preventing the full delivery of wine value during fermentation. The first is sluggish, failing or incomplete fermentations. The second is the production of hydrogen sulfide during fermentation from the presence of elemental sulfur in the must.
He proposed two innovations that can change these fermentation outcomes. The first is real time monitoring, modelling and identification and prediction of fermentations. Then the second, and this is the big one, is the control of redox potential during fermentation. More to the point, with his PhD student James Nelson, who was also at the conference, he has successfully controlled redox potential in commercial-scale ferments. This is the first time this has been achieved.
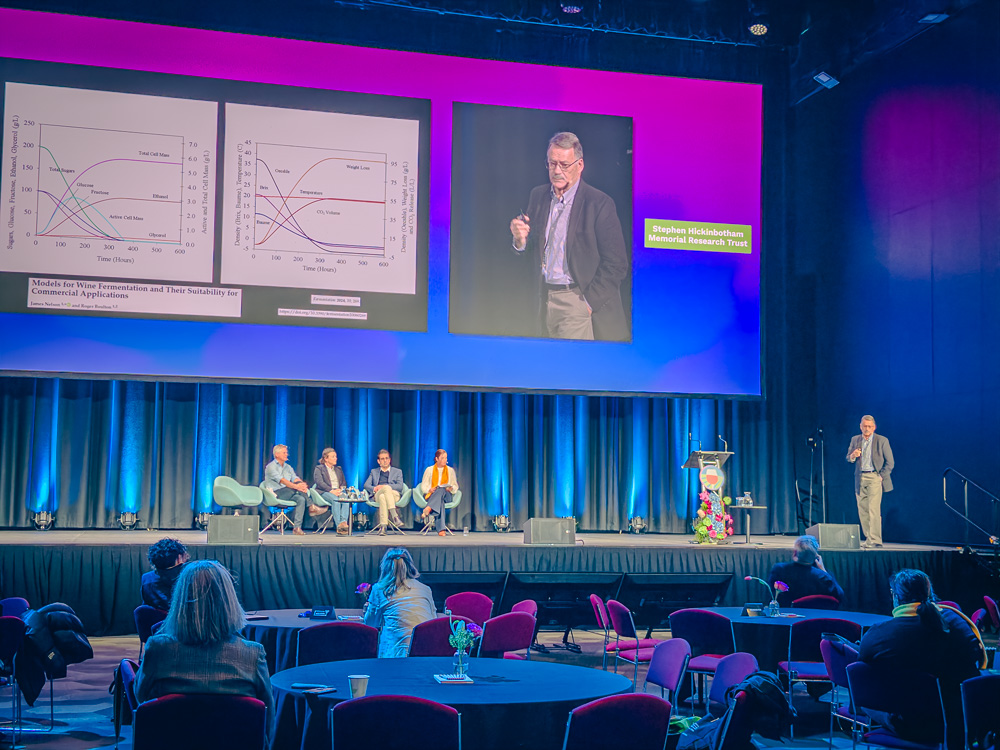
Some explanation is needed here. Redox potential (also referred to as oxidation-reduction potential) is something that can be measured in wine, and it alters the way that reversible chemical reactions take place. But as well as having effects on physical chemistry, it also affects the way that yeast and bacteria behave.
Boulton and Nelson have managed to work out how to track redox reactions in real time during fermentation with a proble mounted on the side wall of a tank. They can see the way that the redox reaction proceeds: normally in a ferment it would start at a certain level and then dip, and then after fermentation has finished it rises again. In these ferments they see that once it dips below -100 mV, then sulfide levels start to rise: hydrogen sulfide is produced, and sometimes at quite high levels. The fall bottoms out at around -180 mV. But redox levels can be held at -50 mV if they use a system to add air at small amounts of around 0.5 mg/litre. This is consumed, but if you add more it isn’t because of the complex chemistry of wine oxidation. There is a small peak in redox level, but when glutathione takes out the peroxide that is formed, then the iron (in the right form) comes back and this peak falls. This process can be repeated as needed. They have done this sort of fermentation control in large ferments, including a 140 000 litre fermentation at Delgats in New Zealand (Sauvignon Blanc), and a 40 000 litre red wine ferment. ‘The impact on fermentation is profound,’ says Boulton.
In concert with this control of redox potential, they also look at how fermentation is proceeding and then compare the data with a model of fermentation performance. This is a predictive model and they can see in real time whether things are going the right way, and if not, then they can interfere. As well as measuring redox potential, they monitor density automatically using pressure transducers, and measure temperature including the skin cap temperature. Often they find that sluggish fermentations are cause by high temperatures. These sluggish fermentations have juice temperatures above 30 C, which means the skin cap is 33 C or so.
This all sounds like groundbreaking work, although I’m sure it will spark some controversy.
The Nelson and Boulton work can be found here: https://www.mdpi.com/2311-5637/11/1/9
Warning: this is pretty hardcore wine chemistry! As an aside, I asked Roger today what the most widely mis-understood aspect of wine chemistry was. His answer, straight back in an instant: oxidation. I think this is a topic I’ll have to ask him more about, as most wine science accounts seem to be wrong on this.




